 Latest
Latest
Japan wouldn't be Japan without their trademark obsession with youth. Here are the top 10 anime starring adorable - and often precocious - kids. Read More
March 8
Shindo Life is a popular Roblox game created by RELL World. Based on the Read More
February 26
How do you get elite passive skills in Palworld? Nintendo may be too safe Read More
January 31 Trending
Trending
The Finals is a new multiplayer gaming experience that pits players against each other Read More
January 31
Here are our picks for the best Sujimon in Like a Dragon: Infinite Wealth. Read More
January 31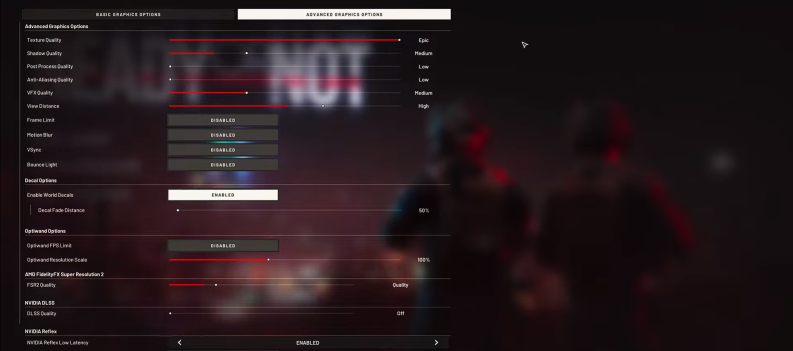
Learn how to improve your FPS in Ready or Not! Ready or Not is Read More
January 30 Gaming News
Gaming News More
More